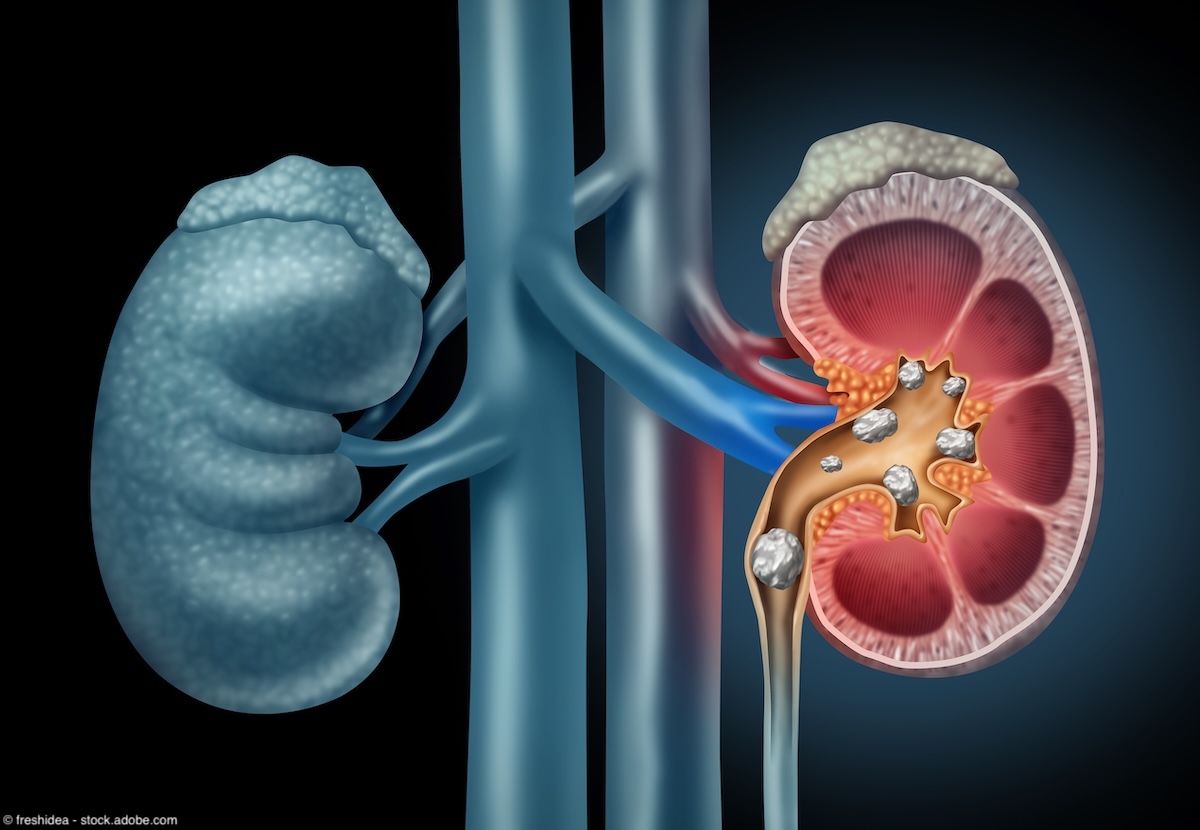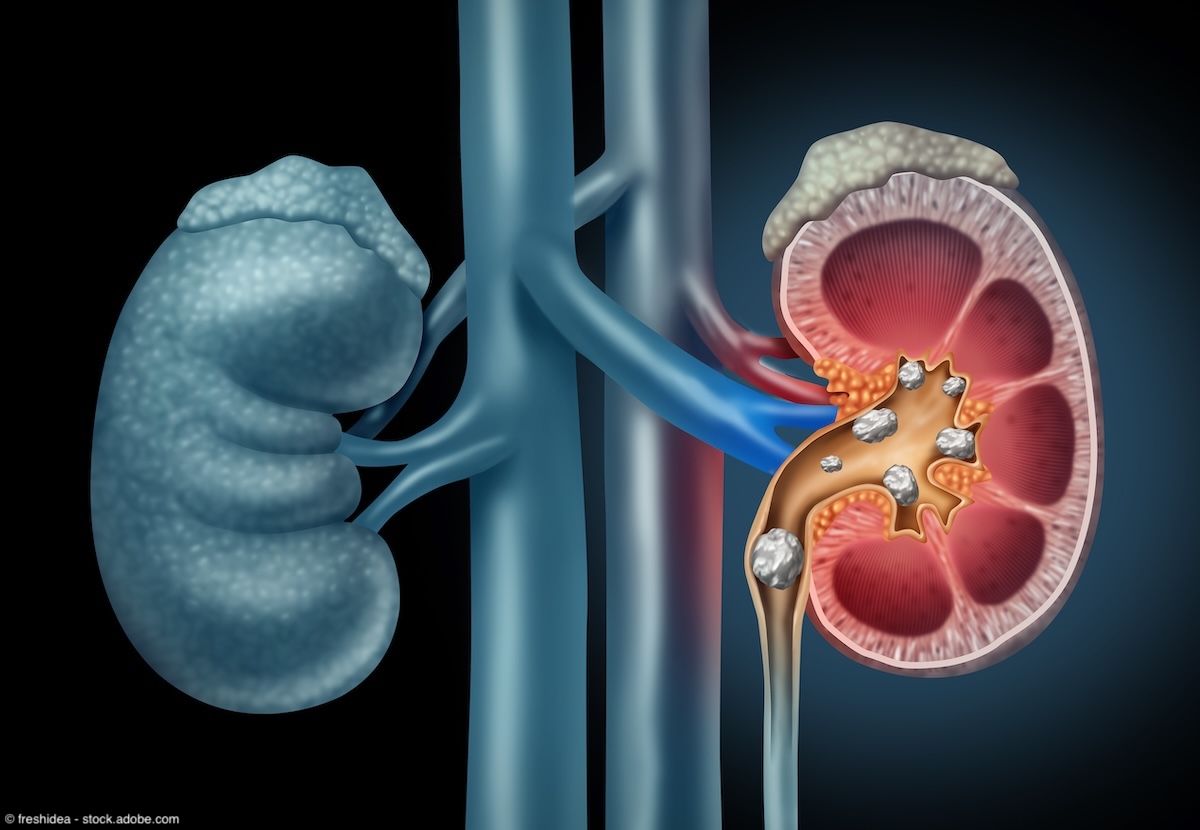
Kidney Stones
Latest News
Video Series

Latest Videos
Shorts



Podcasts
CME Content
More News

A recap of the FDA submissions and regulatory decisions in urology from October 2025.

Avvio Medical plans to submit a de novo application for clearance of the device in early 2026.
Krambeck highlights that FANS combine enhanced maneuverability and effective fragment evacuation, leading to higher stone clearance rates and improved procedural outcomes in endourology.
"Suctioning technology for mini-PCNL is an improvement on relying on passive outflow," says Mantu Gupta, MD.

We recap notable headlines from last month in the benign urology space.
Krambeck pointed to the growing importance of medical management in stone disease prevention.
Margaret A. Knoedler, MD, discusses her experience using the Reverse Deflection CVAC System.

Both the Dornier Hoover Flexible and Navigable Suction Ureteral Access Sheath and the Dornier Axis II Slim single-use ureteroscope are FDA-cleared.

The Focalist device obtained FDA clearance in the US in July 2025.

Two-year outcomes from the ASPIRE trial continued to show that the SURE procedure led to significantly reduced health care consumption events compared with ureteroscopy.

Kevin Koo, MD, MPH, discusses the implications of a digital stone measurement tool for clinical care.

Kevin Koo, MD, MPH, discusses a recent study on the utility of a digital stone measurement tool for ureteroscopy.

We recap notable headlines from last month in the benign urology space.

Joseph Song, MD, discusses his approach to patient selection for the SURE procedure, particularly in comparison to more invasive options such as PCNL.

The findings suggest that urolithiasis continues to pose a significant healthcare burden worldwide.
New findings from the PKIDS trial raise questions about the preference for ureteroscopy in clinical practice.

"What we showed was that there's no difference in the pressures whether you use CVAC or standard ureteroscopy," says Roger Sur, MD.

Joseph Song, MD, details his experience using the CVAC device for SURE procedures.

The first patient has been dosed in the phase 1/2 redePHine trial, evaluating the safety and preliminary efficacy of ABO-101 for PH1.
According to Song, novel aspiration technologies are reshaping not only care delivery, but also long-term treatment outcomes.

The code, 0991T, will become effective on January 1, 2026.

One crucial recommendation is incorporating an ergonomic training module into the curriculum.

Amy E. Krambeck, MD, discusses how to define success and how to determine the optimal timing for adding on a new partner.

David Stanley, MD, FACS, discusses current trends and unmet needs in the treatment of patients with kidney stones.

Margaret A. Knoedler, MD, discusses the advantages of using the MONARCH platform for mini-PCNL.