
According to the authors, these findings suggest that biparametric MRI could become the new standard of care for prostate cancer diagnosis.

According to the authors, these findings suggest that biparametric MRI could become the new standard of care for prostate cancer diagnosis.

Andrew J. Kirsch, MD, FAAP, FACS, outlines current therapies, ranging from behavioral strategies, such as limiting fluids before bedtime, to bedwetting alarms and medications like DDAVP.
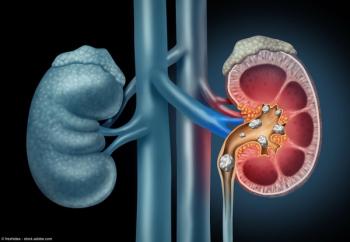
The Focalist device obtained FDA clearance in the US in July 2025.

Tom Jayram, MD, discusses rationale for selective FGFR3 inhibition in bladder cancer as well as what’s needed to bring this treatment modality into routine clinical practice.

Two-year outcomes from the ASPIRE trial continued to show that the SURE procedure led to significantly reduced health care consumption events compared with ureteroscopy.
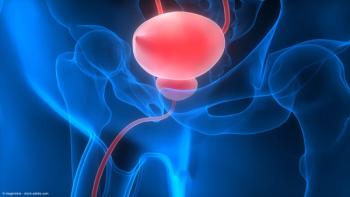
The NDA is supported by data from a phase 3 trial, which demonstrated a 75% complete response rate at 3 months.

Siamak Daneshmand, MD, shares his thoughts on the impact of the gemcitabine releasing system for clinical practice.

The registrational trial is aiming to expand the indications for Illuccix and Gozellix to include use in prostate cancer diagnosis.

Data showed a marked or moderate improvement in IC/BPS symptoms in 41% of patients who received sunobinop vs 9% of patients who received placebo.

"As urologists, we should be proactive and creative as we find new ways to attract, retain and train the next generation," writes Michael S. Cookson, MD, MMHC, FACS.

Data from the SunRISe-1 trial showed a complete response rate of 82%, with 51% of patients remaining in complete response for at least 1 year.

Patients will be enrolled in the study through clinical trial sites in the US, with additional international sites to follow.

The episode also underscores the role of artificial intelligence in prostate imaging.
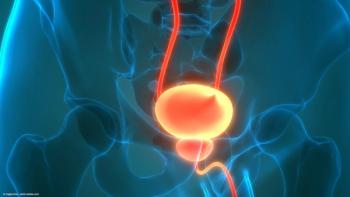
At the time of data report, the median progression-free survival and duration of response had not been reached in either cohort.

"Based on this proposal, some shifts may significantly impact the practice of urology," write Jonathan Rubenstein, MD, and Mark Painter.

Rana R. McKay, MD, FASCO, highlights notable takeaways from the COMRADE trial, assessing the combination of olaparib plus radium-223 in CRPC.

The combination also yielded durable responses in a subset of patients with sporadic papillary renal cell carcinoma.
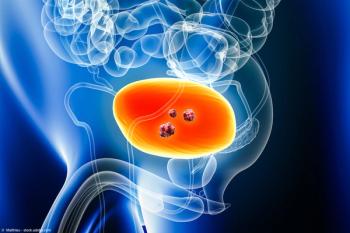
CG Oncology plans to initiate a BLA submission with the FDA in Q4 of 2025.

The AUA has released updated guidelines for recurrent UTIs in women, emphasizing patient-centered care, nonantibiotic options, and improved clinical judgment.

Hong Truong, MD, MS, details key findings on referrals for genetic counseling among patients with kidney cancer who meet the criteria for genetic evaluation.
The biomarker panel demonstrated the ability to detect prostate cancer even in men with prostate-specific antigen levels in the normal range.
The LEGEND trial is evaluating detalimogene voraplasmid in patients with high-risk non–muscle invasive bladder cancer.

The trial is assessing RFS rates with cretostimogene grenadenorepvec vs surveillance following TURBT.

We recap notable headlines from last month in the benign urology space.

The report showed rising incidence rates and a slowing of mortality declines in addition to persistent racial disparities.

Take a look through key stories from last month, including regulatory news, trial updates, and other practice-changing advancements.
Predicine has submitted the first module of a PMA application for their urine cfDNA NGS assay PredicineCARE.

A recap of the FDA submissions and regulatory decisions in urology from August 2025.

The new guidelines provide evidence-based recommendations for treating patients with traumatic injuries to the kidneys, bladder, ureters, urethra, and genitalia.

The approval is supported by findings from an open-label, single-arm, phase 3 trial.