Bladder Cancer
Latest News
Latest Videos

CME Content
More News

The current study is welcome news as it lends further support to the use of intravesical Gem/Doce as a safe and effective option that has the potential to become a new standard of care for high-risk NMIBC.
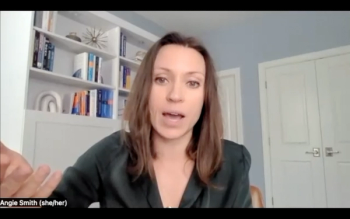
"From a urology provider perspective, we should be discussing this with our patients [with bladder cancer] as they make their treatment decisions," says Angela Smith, MD, MS.

Arya Mariam Roy, MBBS, discusses the effect of neoadjuvant chemotherapy on overall survival in patients with sarcomatoid bladder cancer.

The approval of pembrolizumab plus enfortumab vedotin in urothelial cancer was supported by results from the EV-103/KEYNOTE-869 trial.

“The readers of our paper should really understand that in microhematuria specifically, a lot of the recommendations are based on small amounts of data,” says Jacob Taylor, MD, MPH.

“First, we found that we should not be using voided samples for bladder cancer research,” says Laura Bukavina, MD, MPH.
No CxM-negative patients had findings of a recurrent tumor on a follow-up cystoscopy.

“These shortages and declines in supply have forced practitioners to readjust their diagnostic algorithms or at least question the usual practices,” says Jacob Taylor, MD, MPH.

“If you have 100 bacteria in the voided samples and you have 50 bacteria in the catheterized samples, you assume that a lot of it from the cup is because of contamination,” says Laura Bukavina, MD, MPH.
Roger Li, MD, Moffit Cancer Center, was awarded the grant to study the novel oncolytic virus drug CG0070 in patients with intermediate-risk non–muscle-invasive bladder cancer.
“Microbiome studies are small, and that's a problem. Usually, you have 20 patients here, 30 patients there…We were able to get about 120 patients,” says Laura Bukavina, MD, MPH.

The investigators used data from the National Inpatient Sample database to identify 5280 patients with non-metastatic bladder cancer who underwent RC from 2016 to 2019.

Regardless of the type of chemotherapy regimen received, there was a benefit with the use of frontline avelumab maintenance in patients with locally advanced or metastatic urothelial carcinoma, explains Srikala S. Sridhar, MD.

“There is an urgent need for new treatment options for [patients with] urothelial carcinoma, many of whom find themselves out of options after progressing on immune checkpoint inhibitors," said Sergio Santillana, MD.
Avelumab is approved by the FDA as a maintenance therapy for patients with locally advanced/metastatic urothelial carcinoma that has not progressed with first-line, platinum-based chemotherapy.

“[The study] should provide better insight for clinicians when they're making decisions about treatments and whether surgery is reasonable or not,” says Bernard H. Bochner, MD, FACS.
At 6 months, high-grade RFS in the BCG group was 76%, compared with 92% in the gemcitabine and docetaxel group.

“What we'd like to try to do in our research is see if we can begin to develop a tool that is more personalized for patients, that can provide a more useful approach to quality of life,” says Bernard H. Bochner, MD, FACS.

“When we looked at all the various domains in these 14 different standardized measures…essentially, patients returned to baseline reported levels, usually, by about 1 year or so,” says Bernard H. Bochner, MD, FACS.
Exploratory analysis shows no OS benefit with atezolizumab monotherapy in advanced urothelial cancer
An exploratory analysis of the IMvigor130 study failed to show an improvement in overall survival with atezolizumab, compared with placebo plus platinum-based chemotherapy and gemcitabine in patients with untreated locally advanced or metastatic urothelial cancer.
Treatment with nivolumab in patients with high-risk muscle-invasive urothelial carcinoma continued to improve survival, supporting its use as a standard of following radical resection.
In cohort B of the phase 2 Keynote-057 trial, pembrolizumab led to antitumor activity in patients with BCG-unresponsive, papillary high-risk non–muscle-invasive bladder cancer.

The ORR for patients was 47%. Of note, ORRs were higher in patients with specific alterations compared with wild type including ERBB2 (67% vs. 44%, respectively; P = .05) and TSC1 (68% vs. 25%; P = .04).
In the the phase 2 HCRN GU 16-257 trial, 33 patients with muscle-invasive bladder cancer were eligible to forego cystectomy and continue 240-mg maintenance nivolumab monotherapy every 2 weeks for 8 cycles followed by surveillance.

“There are a couple of nomograms that are good for predicting either T2 or higher disease at time of surgery, so on final pathology, or T3 or higher,” says Suzanne Lange, MD.











