Kidney Cancer
Latest News
Latest Videos

CME Content
More News

"The positive trends emerging from the ongoing phase 1 trial of BMC128, particularly in combination with nivolumab, underscore the transformative potential of microbiome-based therapeutics in oncology,” says Gal Markel, MD, PhD, MBA.
“We may be tempted to conclude a causal relationship based on the results of this analysis; however, we should avoid making statements implying a causal relationship,” says Jaleh Fallah, MD.

"Outside a randomized trial, we cannot make any conclusion about the causal relationship between conducting cytoreductive nephrectomy and improved outcomes," says Jaleh Fallah, MD.
"There’s potentially a role here for patients with small renal masses that may not want to proceed with a minimally invasive laparoscopic or robotic procedure," says Arun Rai, MD, MBA, MSc.
Among the 3 CD70-positive patients, the ORR was 66.7%, with 2 patients achieving a partial response.

The first-in-human study expects to begin dosing patients with DF9001 in combination with pembrolizumab in Q4 of 2024.

The dose escalation/dose expansion study is evaluating the safety and efficacy of AB-2100 in patients with ccRCC who either came back or did not improve following prior treatment with a checkpoint inhibitor and a VEGF inhibitor.
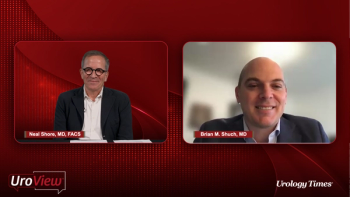
Urologists provide an overview of diagnostic tests for patients with kidney cancer, highlighting lab tests, imaging, and considerations for biopsy.

Neal Shore, MD, FACS, and Brian M. Shuch, MD, introduce themselves and discuss the growing incidence of kidney cancer, the general prognosis, and early signs and symptoms.
“This clinical study has the potential to demonstrate the value of Chimeric Antigen Receptor (CAR) T cell therapy in solid cancers such as kidney cancer with a high unmet medical need,” said Abla Creasey, PhD.
“ARO-HIF2’s ability to target cancer cells, disrupting a key tumor-driving mechanism, illustrates the potential of siRNA technology in oncology,” says James Brugarolas, MD, PhD.

“This is the first study to show a statistically significant and clinically meaningful survival improvement with any adjuvant therapy in kidney cancer, and this further supports adjuvant pembrolizumab as a standard of care after surgery in this disease setting,” says Toni K. Choueiri, MD.

The approval is based on findings from the phase 3 RENOTORCH trial, which showed that toripalimab plus axitinib prolonged progression-free survival and improved the objective response rate in patients with advanced RCC compared with sunitinib.
“Our trial presents the first and the longest durable response we have seen with an allogeneic, off-the-shelf, CAR T cell therapy in the treatment of refractory solid tumors," says Samer A. Srour, MB ChB, MS.

In the monotherapy cohort of TPST-1120, 53% of patients achieved a best response of stable disease, with 5 of those patients remaining on treatment for more than 5 months.
Treatment with stereotactic ablative body radiotherapy resulted in no observed local failures or cancer-related deaths among patients with primary renal cell cancer.

“The take-home message is: Don’t assume bad things if the tumor is growing during chemotherapy," says Andrew M. Davidoff, MD.

With the dosing of the first patient in Italy, Telix’s expanded access program for TLX250-CDx in ccRCC now includes 3 active countries, including the Netherlands and the US.

The VISTA-101 study has dosed all patients at dose level 5 of 6 in the KVA12123 monotherapy arm and all patients at the dose level 2 of 4 in the KVA12123-pembrolizumab combination arm.

“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer," says Han-Ping Shi, MD, PhD.

"I emphasized the fact that the use of ultrasound is very important and should be done on every single case," says Nazih Paul Khater, MD, FACS.

The FIT-001 trial is assessing KO-2806 as a monotherapy and in combination with targeted therapies in patients with advanced solid tumors, including ccRCC.

The study plans to enroll 144 patients with solid tumors, including RCC, non-small cell lung cancer, colorectal carcinoma (CRC), and other malignancies known to express HHLA2/B7-H7.

“These clinical data show encouraging safety and efficacy with JANX007 in metastatic castration-resistant prostate cancer and with JANX008 in late-stage solid tumors," says David Campbell, PhD.

In total, the investigators assessed data from 886 patients with advanced kidney cancer who were randomly assigned to receive avelumab plus axitinib or subitinib.